Part 1 – Overview
1.1 Purpose and Structure of the IHerbSpec Protocol
This document outlines a “base” protocol for the spectroscopic measurement of herbarium tissues. It defines clear minimum requirements and recommended practices that promote data quality, methodological transparency, and interoperability across projects and institutions. In addition to these foundations, the protocol has been expanded to incorporate broader discussions surrounding instrumentation, materials, and herbarium tissues to further guide best practices.
The protocol specifies two categories of procedural elements:
- Minimum requirements – essential components to enable data harmonization that must be met for a project to be considered aligned with the IHerbSpec Protocol.
- Recommended practices – additions that enhance data quality and support broader utility, but are not mandatory.
The IHerbSpec Protocol is organized into modular parts:
- Part 1 – Protocol Overview
- Part 2 – Measurement and Metadata Workflow
- Part 3 – Filename Conventions and Formats
- Part 4 – Metadata and Databasing
- Part 5 – Instrumentation and Materials Guidelines
- Part 6 – Selecting Tissues for Spectral Measurement
- References
- Appendix I – Number of Measurements per Tissue
- Appendix II – Tissue Metadata for Quality Control
While consistency is central to aggregating spectral datasets, the protocol is not intended to prescribe a rigid workflow. The core requirements are designed to enable data harmonization through methodological consistency and transparency, while the overall structure preserves the flexibility needed for its broad adoption, continued innovation, and refinement.
1.2 Measurement and Metadata Overview
This section provides a quick conceptual guide to the required and recommended components of the protocol with relevant notes on adaptability.
1.2.1 Filename Conventions
To ensure key identifiability components for every unprocessed spectra file (white target, black target, calibrated reflectance standards, tissues), the IHerbSpec Protocol strongly recommends using the filename formats described in Part 3.
Adaptability: Projects may use simplified filenames (see Table 3.1) during metadata scoring, then convert to the full format for permanent storage and distribution.
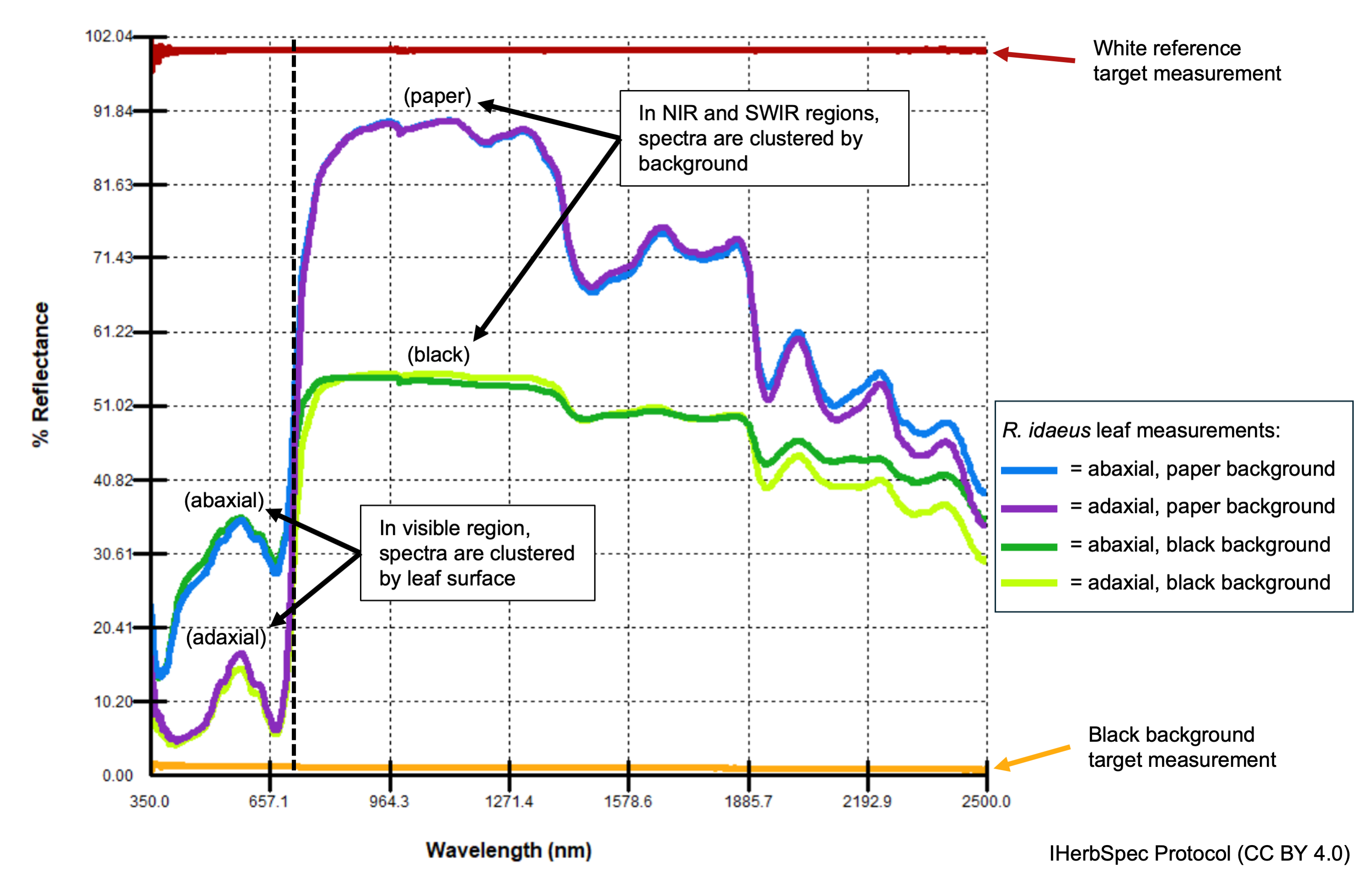
1.2.2 White Reference and Background Measurements
White-reference measurements are required at the start of each session and at regular intervals (e.g., every 20 minutes; check manufacturer guidance).
- This measurement is not recorded as a unique file (see Section 5.3).
- A measurement session is defined as the period between switching the instrument on and off.
White, black, and/or gray calibrated reflectance standard target measurements are recommended to be taken at the beginning of each measurement session or at regular intervals (e.g. once per week) and be locally archived (see Section 5.3).
A single white target measurement is required at the beginning of each session to confirm instrument performance (see Fig. 1.1).
- It is strongly recommended to use the
sessionIdfield in filenames in order to link the white-reference spectrum to every tissue measurement in the session (see Table 4.1).
- It is strongly recommended to use the
The protocol requires projects to use a <4% reflective black surface whenever possible as a background behind tissues during measurement (Section 5.3).
If tissues are glued and must be measured against herbarium sheet paper as the background, then a paper background target measurement must be taken and linked to the corresponding tissue spectra (Fig. 1.1; Part 3).
1.2.3 Strategy and Number of Tissue Measurements
Tissue selection strategy
The number of leaf tissue measurements per specimen
- If both adaxial and abaxial leaf surfaces are accessible and suitable, a minimum of three representative measurements per surface (six total) is required per specimen. If only one surface is available or if the tissue class is
leafwith undifferentiated abaxial/adaxial surfaces (see Table 4.5), then a minimum of three representative measurements are required.- Representative measurements should be free of technical errors and generally reproducible (see Section 6.2; Appendix I).
- Representative measurements should be free of technical errors and generally reproducible (see Section 6.2; Appendix I).
- Five or more representative measurements per available and suitable leaf tissue class (adaxial, abaxial, or leaf) are recommended (see Appendix I for discussion).
Adaptability: Projects may choose to collect measurements from a single leaf or from multiple leaves to capture tissue- versus specimen-level variability. The different tissue units are recommended to be distinguished using the
targetTissueIdfield and be identifiable via annotation labels and/or their location on the specimen (see Table 4.3).- If both adaxial and abaxial leaf surfaces are accessible and suitable, a minimum of three representative measurements per surface (six total) is required per specimen. If only one surface is available or if the tissue class is
Other tissue classes
- No required minimum is prescribed.
- Recommended: At least three representative measurements per specimen per tissue class, if feasible.
- No required minimum is prescribed.
1.2.4 Measurement Technique
- Multiple measurements per tissue unit may be taken by targeting multiple suitable areas on the tissue or—taking care not to heat-damage the tissue—by targeting a small area and slightly rotating or shifting the optical probe between measurements.
- Avoid overlapping tissue layers, debris, or partial coverage of the probe aperture (Fig. A1).
- If a measurement appears affected by technical error, delete and repeat it.
1.2.5 Metadata Requirements and Flexibility
- All required and recommended protocol metadata are available for use in the IHerbSpec Metadata Spreadsheet, which can be downloaded on the protocol home page.
- The protocol recommends recording tissue metadata for every individual measurement because every measurement will generate a spectral data file. This means that every tissue measurement will be recorded in a unique row in the metadata spreadsheet (e.g., 10 measurements have 10 rows).
- All fields in Session metadata (Table 4.1), Specimen Metadata (Table 4.2), and Tissue Metadata (Table 4.3) are marked as required or recommended in the field descriptions.
1.2.6 Scoring Metadata Pertaining to Tissue Condition and Contamination
- To record key elements of tissue condition and contamination, these fields are required metadata (see Section 6.1; field descriptions in Table 4.3):
tissueDevelopmentalStage
hasGlue
hasNonGlueContamination
- To further describe tissue conditions, the
measurementFlagsandtissueNotesfields are recommended:measurementFlagsallows use of standardized tissue condition descriptors (see Table 4.6) for preservation status, damage, or contamination (e.g.,GoodPreservation|GluePresent).
tissueNotesenables free-text documentation of additional tissue conditions or context inside or outside the measurement area.
Adaptability:
tissueDevelopmentalStagemay be coded asnotScoredif assessment is not possible (e.g., due to technician training or specimen condition; see Table 4.4).
hasGlueandhasNonGlueContaminationmay be markeduncertainif presence is not confidently determined.
1.2.7 Specimen and Tissue Annotation
- The IHerbSpec Protocol recommends annotating the location of measured tissues on the sheet using the
targetTissueIdfield. Additional annotations to the herbarium sheet or packet are encouraged in accordance with herbarium policies (see Section 5.4).