Part 5 – Instrumentation and Materials Guidelines
5.1 Precautions
5.1.1. Carefully handle backgrounds and white references (e.g., Spectralon®):
- Use laboratory gloves to handle black backgrounds and white references and tweezers to handle herbarium tissues.
- Avoid touching and dirtying the reflective surfaces.
- Avoid blowing or cleaning backgrounds and reference standards with canned air, which can cause chemical contamination (other options, such as O2 Hurricane, avoid this contamination).
5.1.2. Avoid burning target tissues:
- High light intensities in optical probes can burn tissues, especially thin leaves! Use low light intensity and minimum measurement duration based on manufacturer’s guidance to avoid this.
- Avoid measuring tissues with glue on them, whenever possible.
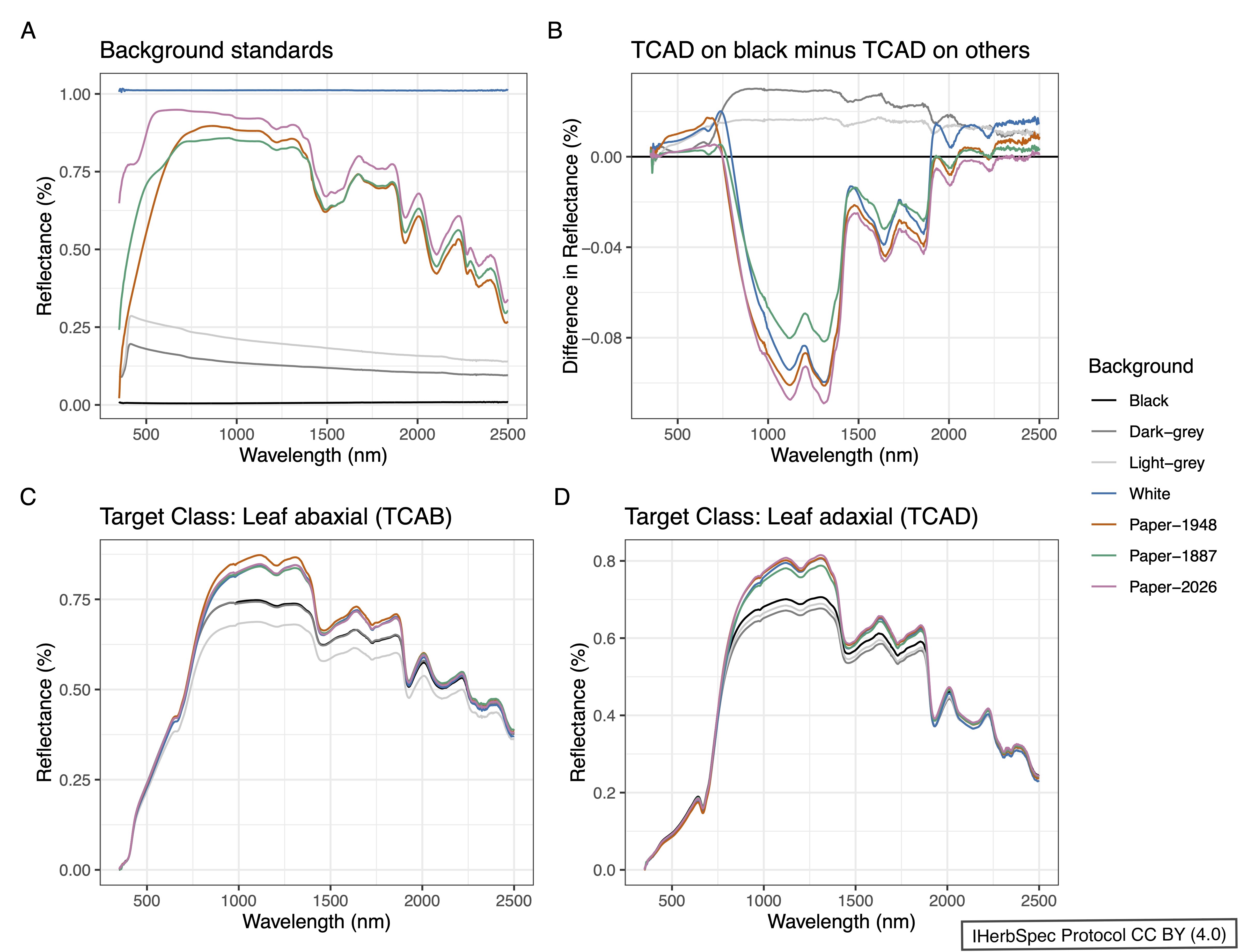
- Place a black background under the leaf or target tissue to avoid spectral contamination from the herbarium mounting paper whenever possible. Background surfaces, except for non-reflective black, will influence reflectance spectra (see Fig. 1.1).
5.2 Spectra Data Files
5.2.1. Output unprocessed percent reflectance data to files, if possible.
- Percent reflectance should be present in the file at the wavelengths directly measured by the instrument.
- If instruments and software output transformed data by default (e.g., Spectral Evolution PSR+ output is interpolated at 1 nm resolution), refer to user manuals to change settings to output percent reflectance values at measured wavelengths instead.
5.2.2. File formats differ among spectroradiometer manufacturers, models, and softwares.
- Make sure that files include percent reflectance values.
5.3 Instrumentation and Materials Quality Control
5.3.1. Instrument maintenance:
- Follow manufacturer SOP for spectroradiometer maintenance. If possible, send the instrument to the manufacturer at regular intervals for maintenance (e.g., every two years).
- Have fiber optic cables checked by the manufacturer annually and replace damaged fiber optic cables. You can test the integrity of the cables by shining a flashlight through one end of the fiberoptic and observing the light transmitted out the other side.
5.3.2. For background and white reference measurement requirements, see Section 1.2.2.
- Background materials are not spectrally neutral and exhibit distinct reflectance profiles that can introduce systematic, wavelength-dependent biases into leaf spectral measurements (Fig. 5.1). These effects are especially pronounced in the near-infrared and shortwave infrared regions, underscoring the importance of consistent background selection and documentation for reproducible and comparable spectral data.
- White reference scans are typically not saved as a separate file, but the white reference digital numbers (raw sensor values) should be included in each target spectrum file (e.g.,
.sigformat). These values are used by the instrument software to calculate reflectance from raw radiance.
- Black background materials are required to have <4% reflectance across all wavelengths (see Fig. 1.1; e.g., IR Flock Sheet from Musou Black USA).
- If measuring leaves on herbarium paper on a benchtop, place a black mat under the herbarium sheets to prevent contaminating reflectance from the benchtop (see Fig. 4.2). Black felt (3–5 mm thick) is a practical and cost-effective choice for this purpose.
5.3.3. Target measurements of background material (black or paper) must be linked to tissue measurements using filename conventions (Part 3).
- This supports quality control of background conditions and enables development of spectral unmixing approaches to isolate tissue reflectance.
5.3.4. Wear gloves and carefully handle all tissues, white reference, black backgrounds, and calibrated reflectance standards to keep them clean (see 5.1 Precautions).
5.3.5. Calibrated reflectance standards:
- Institutions should consider purchasing calibrated reflectance standards, such as Spectralon® Calibrated (SL Standard) Diffuse Reflectance Standards. These should be kept clean and separate from ‘working’ white references, and used for long-term assessment of instrument performance, optical drift, and the condition of reference materials (e.g., white references and black backgrounds).
- These are supplied with a calibration certificate and serial number that verifies reflectance values across wavelengths.
- Reflectance standards are white (e.g., Spectralon® 99%) and black (e.g., Spectralon® 2%) at a minimum, and can also include gradations of gray.
- It is recommended that standards be measured at regular intervals depending on the level of activity (e.g., once per session; consult spectroradiometer manufacturer), and archived with the filename conventions provided in Table 3.1.
5.4 Specimen and Tissue Annotation
5.4.1. Metadata recommendation:
- IHerbSpec recommends recording the
tissueLocationmetadata field (see Table 4.3) to document the position of measured tissues on the specimen.
5.4.2. Consult with herbarium collections managers to define a suitable specimen annotation protocol that aligns with institutional policies.
5.4.3. Recommended specimen annotation practices:
- Attach a project annotation label to the herbarium sheet (e.g., “Leaf spectral reflectance; DM White (2025)”).
- Use archival-quality materials for labels and annotations, such as acid-free paper.
- Use pencil for direct annotation of measured tissues on sheets.
- For loose tissues (from packets or envelopes):